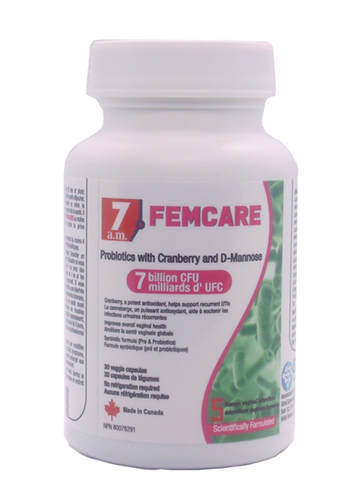
Product Description
Composition:
Medicinal Ingredients:
Each Capsule contains
Lactobacillus acidophilus La14,Lactobacillus rhamnosus Lr32,Lactobacillus paracasei Lpc37, Lactobacillus plantarum Lp115,Lactobacillus salivarius Ls33,Cranberry fruit juice powder -500 mg,D-Mannose-100 mg, Fructooligosaccharide 30 mg
Total:7 Billion CFU
Recommended Use:
- Helps to prevent recurrent UT infection.
- Improves overall vaginal health
- A factor in the maintenance of good health
- Anti Oxidant
Directions for Use:
Take 1 to 2 capsules at a time with a glass of water. If on antibiotic(s), use 2 to 3 hours after or before.
Recommended Dose:
Adults (18 Years and Above) Take 1 Capsule before breakfast in the morning as a dietary supplement or as recommended by health care practitioner.
Caution(s) and warning(s)
- If you have fever, vomiting, bloody diarrhea or severe abdominal pain, consult a health care practitioner prior to use.
- If symptoms of digestive upset (e.g. diarrhea) occur, worsen, or persist beyond 3 days, discontinue use and consult a health care practitioner.
Contraindication(s)
- If you have an immune-compromised condition (e.g. AIDS, lymphoma, patients undergoing long-term corticosteroid treatment), do not use this product.
Storage: Store in a cool and dry place
Note: For Oral Use Only
METERIAL SAFETY DATA SHEET
1. INDENTIFICATION
LABEL NAME: 7 AM FEM CARE PROBIOTICS CAPSULES 30 CT
TRADE NAME: SAME FEMA NUMBER: NONE CAS NUMBER: NONE
2. PHYSICAL DATA
DESCIPTION: ODORLESS, WHITE CAPSULES
SOLUBILITY IN H2O: SOLUBLE SPECIFIC GRAVITY: N/A
STABILITY: STABLE IN RECOMMENDED STORAGE CONDITION
3. FIRE, EXPLOSION AND REACTIVITY DATA
FLASH POINT: N/A DOT HAZARD CLASSIFICATION: N/A
NFPA HAZARD: N/A
EXTINGUISHING MEDIA: N/A
SPECIAL FIREFIGHTING PROCEDURES: NONE
UNUSUAL FIRE AND EXPLOSION HAZARDS : DUST FROM POWDER MAY BE EXPLOSION HAZARD IN VERY LARG QUANTITIES.
HAZARDOUS COMBUSTIBLE OR DECOMPOSITION PRODUCT: BURNING CAN PRODUCE CARBON DIOXIDE AND/OR CARBON MONOXIDE.
CONDITIONS AND MATERIAL TO AVOID: NONE
HAZARDOUS POLYMERIZATION PRODUCTS: NONE
4. PROTECTION INFORMATION
RESPIRATORY: AVOID PROLOGED EXPOSURE; WEAR POWDER MASK AS A PRECAUTION.
VENTILATION: NORMAL MECHANICAL VENTILATION.
EYE: CHEMICAL SAFETY GOGGLES RECOMMENDED AS A PRECAUTION.
SKIN: RECOMMEND WEARING PROTECTIVE GLOVES.
OTHER PROTECTIVE DEVICES AND PROCEDURES: FOLLOW GOOD MANUFACTURING PROCEDURES.
5. OCCUPATIONAL EXPOSURE LIMIT
THERSHOLD LIMIT VALUE (TLV): NOT ESTABLISHED
OSHA PERMISSIBLE EXPOSURE LIMIT (PEL): NOT ESTABLISHED
6. HAZARD INFORMATION
HEALTH HAZARD DETERMINATION:
NO CHRONIC HEALTH HAZARD INFORMATION IS AVAILABLE.
7. EMERGENCY AND FIRST AID PROCEDURES
INHALATION EXPOSURE: REMOVE TO FRESH AIR.
EYE CONTACT: REMOVE CONTACT LENSES. RINSE EYES THOROUGHLY WITH WATER. IF NECESSARY, SEEK MEDICAL ATTENTION.
SKIN CONTACT: WASH EXPOSED AREAS WITH A MILD SOAP AND PLENTY OF WATER.
OTHER: NONE
8. SPILL, LEAK AND DISPOSAL PROCEDURES
PRECAUTIONS IF MATERIAL IS SPILLED OR RELEASED:
SMALL SPILL: SWEEP AREA, REMOVE TO DISPOSAL AREA.
LARGE SPILL: SWEEP AREA, REMOVE TO DISPOSAL AREA.
WASTE DISPOSAL METHODS : DISPOSE OF PROPERLY IN ACCORDANCE WITH LOCAL, STATE AND FEDERAL REGULATIONS.
9. HANDLING AND STORGE PROCEDURES
STORE AT ROOM TEMPERATURE (BELOW 25 DEGREE CELSIUS), DRY AREA IN TIGHTLY SEALED CONTAINERS.
10. CHEMICAL AND COMMON NAMES
PROBIOTICS OR LACTIC ACID BACTERIAS