Product Description
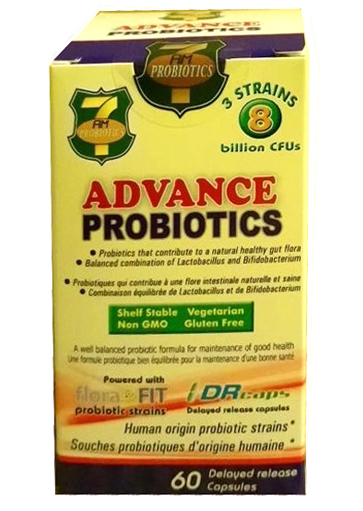
Product Name: 7 AM Advance Probiotic Capsules
Description: 16.2 B CFU/G, White, Odorless, Size‘0’ delayed release veggie capsules.
Composition:
Active Ingredients: Bifidobacterium lactis BL04, Lactobacillus acidophilus LA14, Lactobacillus rhamnosus LR32
Other Ingredients: Hypromellose, Rice Maltodextrine, Fructooligosaccharide, Vegetable grade magnesium stearate.
Use: Source of Probiotics, Maintains healthy intestinal flora.
Dose: 2 capsules in morning before breakfast for adults and adolescents of age 12 years and over.For childrens of 6 to 12 years age 1 capsule in morning before breakfast.
Packaging:30& 60 CT Capsules in HDPE Bottle.
Storage: Store in a cool and dry place. May refrigerate after opening to maintain freshness.
Shelf Life: 24 months from the date of manufacturing under recommended storage condition
Country of Origin: Produce of Canada. Probiotic cultures are of US Origin
Regulatory Status: Approved by Health Canada
NPN: 80044183
METERIAL SAFETY DATA SHEET
1. INDENTIFICATION
LABEL NAME: 7 AM ADVANCE PROBIOTICS CAPSULES 60 CT
TRADE NAME: SAME FEMA NUMBER: NONE CAS NUMBER: NONE
2. PHYSICAL DATA
DESCIPTION: ODORLESS, WHITE CAPSULES
SOLUBILITY IN H2O: SOLUBLE SPECIFIC GRAVITY: N/A
STABILITY: STABLE IN RECOMMENDED STORAGE CONDITION
3. FIRE, EXPLOSION AND REACTIVITY DATA
FLASH POINT: N/A DOT HAZARD CLASSIFICATION: N/A
NFPA HAZARD: N/A
EXTINGUISHING MEDIA: N/A
SPECIAL FIREFIGHTING PROCEDURES: NONE
UNUSUAL FIRE AND EXPLOSION HAZARDS : DUST FROM POWDER MAY BE EXPLOSION HAZARD IN VERY LARG QUANTITIES.
HAZARDOUS COMBUSTIBLE OR DECOMPOSITION PRODUCT: BURNING CAN PRODUCE CARBON DIOXIDE AND/OR CARBON MONOXIDE.
CONDITIONS AND MATERIAL TO AVOID: NONE
HAZARDOUS POLYMERIZATION PRODUCTS: NONE
4. PROTECTION INFORMATION
RESPIRATORY: AVOID PROLOGED EXPOSURE; WEAR POWDER MASK AS A PRECAUTION.
VENTILATION: NORMAL MECHANICAL VENTILATION.
EYE: CHEMICAL SAFETY GOGGLES RECOMMENDED AS A PRECAUTION.
SKIN: RECOMMEND WEARING PROTECTIVE GLOVES.
OTHER PROTECTIVE DEVICES AND PROCEDURES: FOLLOW GOOD MANUFACTURING PROCEDURES.
5. OCCUPATIONAL EXPOSURE LIMIT
THERSHOLD LIMIT VALUE (TLV): NOT ESTABLISHED
OSHA PERMISSIBLE EXPOSURE LIMIT (PEL): NOT ESTABLISHED
6. HAZARD INFORMATION
HEALTH HAZARD DETERMINATION: NO CHRONIC HEALTH HAZARD INFORMATION IS AVAILABLE.
7. EMERGENCY AND FIRST AID PROCEDURES
INHALATION EXPOSURE: REMOVE TO FRESH AIR.
EYE CONTACT: REMOVE CONTACT LENSES. RINSE EYES THOROUGHLY WITH WATER. IF NECESSARY SEEK MEDICAL ATTENTION.
SKIN CONTACT: WASH EXPOSED AREAS WITH A MILD SOAP AND PLENTY OF WATER.
OTHER: NONE
8. SPILL, LEAK AND DISPOSAL PROCEDURES
PRECAUTIONS IF MATERIAL IS SPILLED OR RELEASED:
SMALL SPILL: SWEEP AREA, REMOVE TO DISPOSAL AREA.
LARGE SPILL: SWEEP AREA, REMOVE TO DISPOSAL AREA.
WASTE DISPOSAL METHODS : DISPOSE OF PROPERLY IN ACCORDANCE WITH LOCAL, STATE AND FEDERAL REGULATIONS.
9. HANDLING AND STORGE PROCEDURES
STORE AT ROOM TEMPERATURE (BELOW 25 DEGREE CELSIUS), DRY AREA IN TIGHTLY SEALED CONTAINERS.
10. CHEMICAL AND COMMON NAMES
PROBIOTICS OR LACTIC ACID BACTERIAS